A newly developed portable capillary liquid chromatograph was investigated for the separation of various pharmaceutical and illicit drug compounds. The system consists of two high-pressure syringe pumps capable of delivering capillary-scale flow rates at pressures up to 10,000 psi. Capillary LC columns packed with sub-2 μm particles are housed in cartridges that can be inserted into the system and easily connected through high-pressure fluidic contact points by simply applying a specific, pre-determined torque rather than using standard fittings and less precise sealing protocols. Several over-the-counter analgesic drug separations are demonstrated, along with a simple on-line measurement of tablet dissolution. Twenty illicit drug compounds were also separated across six targeted drug panels. The results described in this study demonstrate the capability of this compact LC instrument to address several important drug-related applications while simplifying system operation, and greatly reducing solvent usage and waste generation essential for on-site analysis.
Keywords: capillary liquid chromatography, portable, pharmaceuticals, illicit drugs, dissolution monitoring
1. Introduction
Liquid chromatography (LC) continues to be one of the most important techniques for the chemical analysis of pharmaceutical, forensic, food and beverage, environmental, biomedical, and clinical samples. Although commercial LC instrumentation has gradually improved over the past five decades, except for the pronounced development of ultrahigh pressure liquid chromatography (UHPLC) since 2004 [1], the general approach to the technique has remained fundamentally the same over this time period. In contrast to gas chromatography (GC), the use of capillary-scale columns has not dominated the LC field during its maturation. Rather, capillary LC has been primarily utilized in ‘omics’ applications because of its enhanced sensitivity when interfaced to mass spectrometry (MS) as compared to analytical-scale columns operated at much higher flow rates [2–4]. The key advantages of capillary LC include (1) reduced flow rate, which makes the method greener compared to analytical-scale LC, and (2) improved heat dissipation, which eliminates the effects of viscous friction when coupled with lower flow rates [5,6]. However, concerns about column robustness and reduced sensitivity using optical detection modes have limited the use of capillary LC much beyond the aforementioned ‘omics’ studies [7,8].
In this Highlights article, a novel approach to capillary LC instrument design is introduced to alleviate some of these concerns. To realize this new design, advances in small, high-pressure LC pumps [9–11] and on-column UV-absorbance detection (LC-UV) [12,13] have been integrated into a compact instrument footprint that is field-portable [14]. Column robustness issues and extra-column band broadening related to poor connections in capillary systems are reduced through the use of a novel cartridge design that applies appropriate torque for fluidic connections at pressures up to 10,000 psi. This is in contrast to other portable LC systems that employ more traditional LC column integration and lower pressure limits [15–18]. This first report demonstrating the use of the new, compact LC system addresses several common applications in the pharmaceutical and forensic fields.
2. Discussion
2.1. Description of the Compact Capillary LC Platform
An overall schematic of the instrument design of the portable capillary LC platform is shown in Figure 1. The system is 20.1 cm (height) × 23.1 cm (width) × 32.0 cm (depth) and weighs 7.82 kg (a size comparison to other portable LC systems can be found in the Supporting Information). Two high-pressure syringe pumps capable of delivering flow rates of 0.8–50 μL/min at pressures up to 10,000 psi are operated in tandem and connected to a mixing valve so that a single mobile phase with combined solvents from two reservoirs is delivered to the injection valve (either in isocratic or gradient operating modes). The injector contains an internal sample loop with 40 nL volume (this value can be decreased to as low as 4 nL with a different internal loop or increased up to 700 nL with a slightly modified valve), which follows a mobile phase mixing valve and connects to the cartridge through a short section of small-diameter (i.e., 75 μm i.d.) tubing. The capillary LC column (fused silica with a UV-transparent outer coating packed with sub-2 μm stationary phase particles) is mounted inside a cartridge that also contains an on-capillary UV-absorbance detector with a light-emitting diode (LED) light source [13,19]; the inlet of the capillary forms a high-pressure fluidic connection to the tubing from the injector when torque is applied to the cartridge mechanism from a calibrated screw knob on the front of the instrument. In this study, the cartridges contained 100 mm × 150 μm i.d. capillary columns packed with sub-2 μm C18 fully porous particles. Applications that are commonly performed with traditional LC instrumentation were translated to the capillary scale and performed using the integrated, compact platform.
Figure 1.
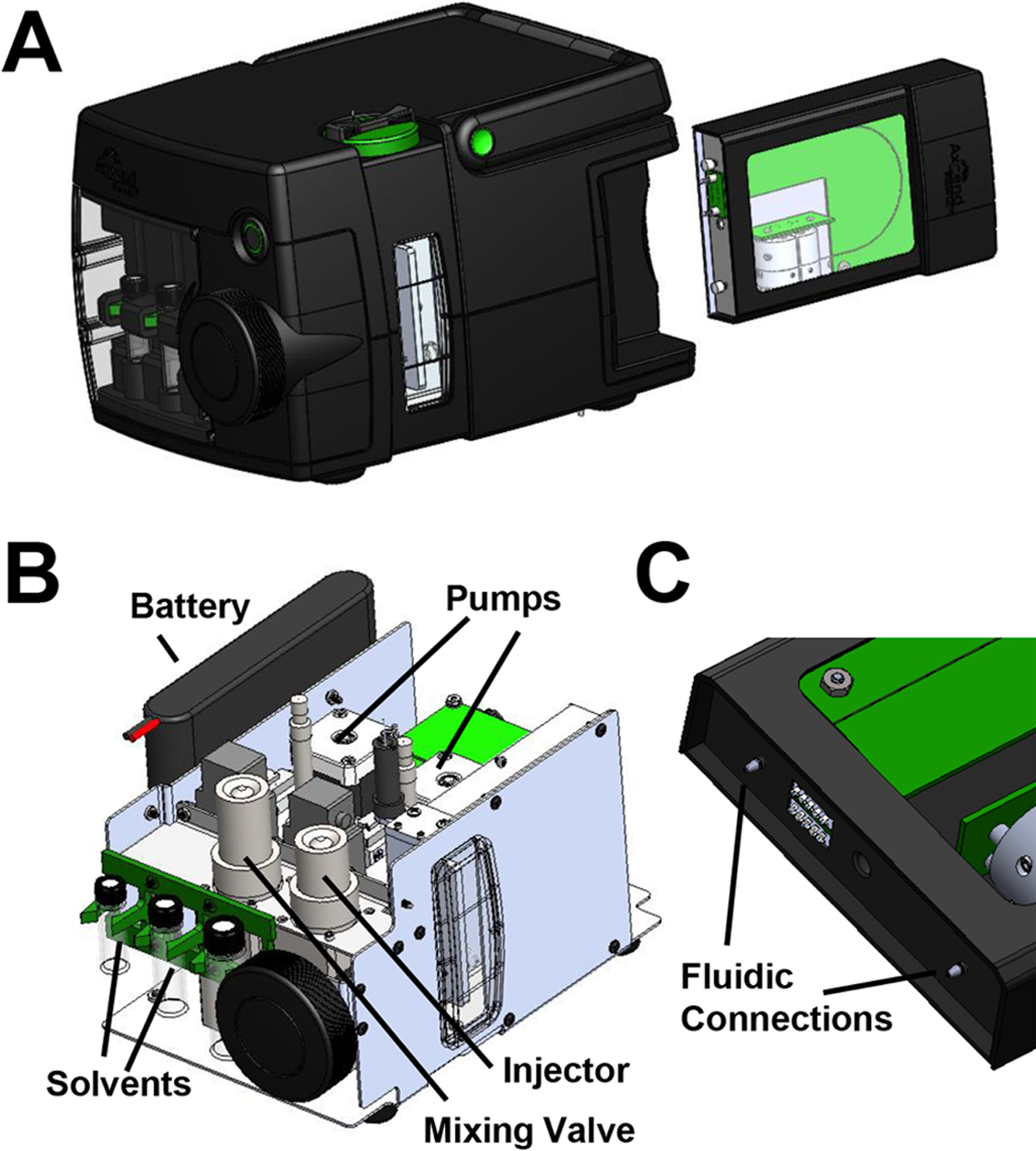
3D diagram of the complete portable LC instrument with removable capillary column cartridge is shown in panel (A). An internal drawing depicting various essential components in the system is shown in panel (B). The fluidic connections that are formed when the cartridge is inserted into the instrument and torque is applied are highlighted in panel (C).
2.2. Use of the Compact Capillary LC Platform for Pharmaceutical Separations
One key area that regularly employs LC separations coupled to UV-absorbance detection is the analysis of small molecule drugs in the pharmaceutical industry. In Figures 2a–c, separations of common over-the-counter (OTC) analgesic drugs are demonstrated based on methods described in the United States Pharmacopeia-National Formulary (USP-NF) [20–22]. These techniques were not directly adapted from previously published monographs using guidelines established in USP Chapter 621 [23,24], but rather developed using faster gradient LC approaches that are more common in the pharmaceutical industry for method screening. Ongoing investigations are focused on determining the full linear range of these separations and determining the applicability of the technique at trace impurity levels (0.05% of the active pharmaceutical ingredient, or API). In general, on-column detection provides shorter path-lengths than typical flow cells, which can decrease sensitivity; however, this approach was used to avoid significant extra-column band broadening.
Figure 2.
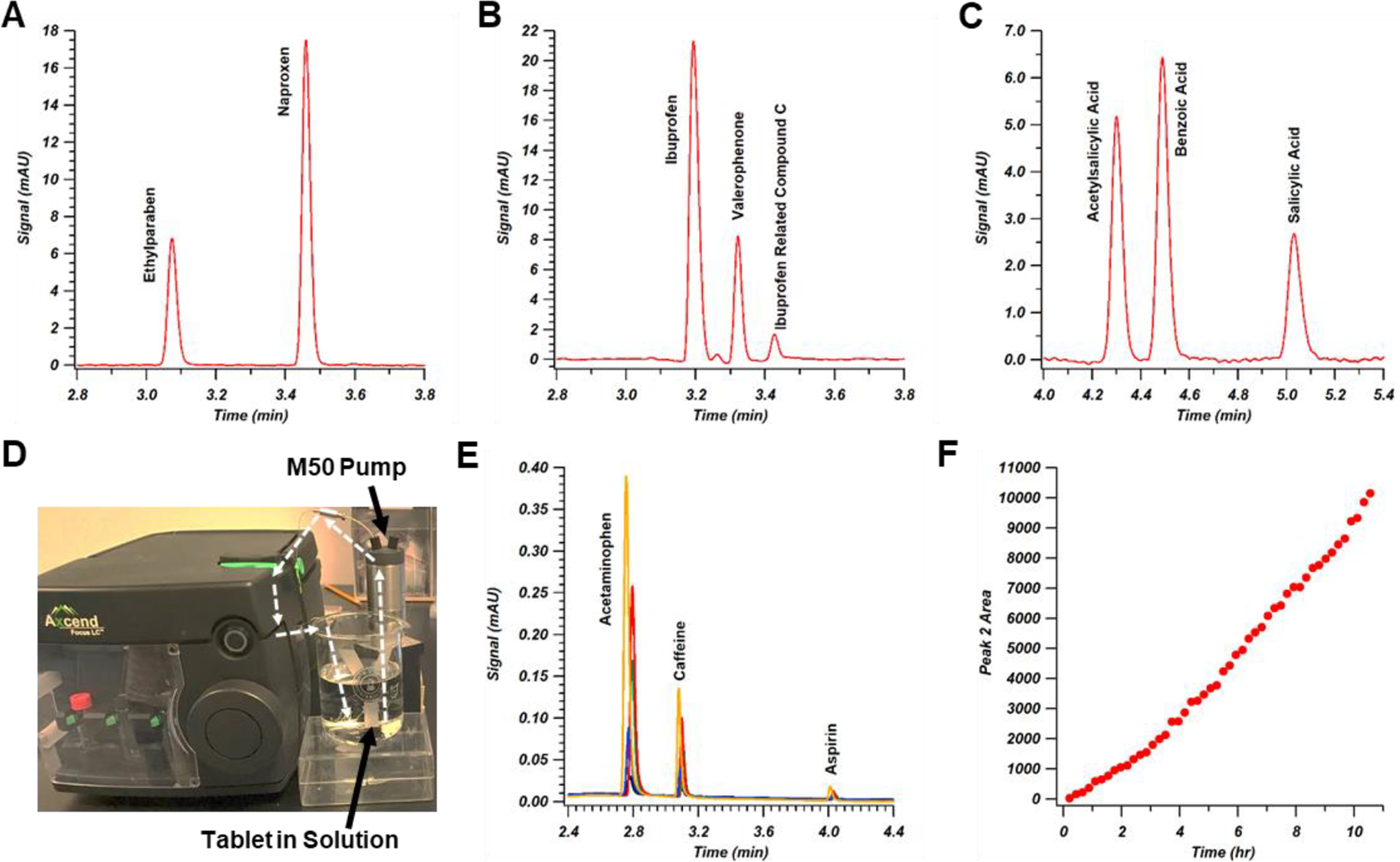
Pharmaceutical applications of the portable LC instrument. Analytical methods for over-the-counter analgesic drugs naproxen, ibuprofen, and acetylsalicylic acid (i.e. aspirin) are shown in panels (A-C). Panel (D) depicts the use of a M50 pump to circulate a tablet dissolution vessel through the injection port for on-line repeated analysis (general flow path shown with white dashed arrows). Selected chromatograms after 10 runs (black trace), 20 runs (blue trace), 30 runs (green trace), 40 runs (red trace), and 50 runs (orange trace) are shown in panel (E), with acetaminophen, caffeine, and aspirin peaks resulting from tablet dissolution. In panel (F), the calculated area for the caffeine peak is shown for all 50 runs (~13 min cycle time per run). Further experimental details can be found in the Supporting Information.
Beyond standard API and impurity testing, dissolution and process monitoring studies are also critical aspects of pharmaceutical analysis. By coupling the capillary LC platform directly to a solution vessel, these types of measurements can be made on-line, which eliminates the need for manual sampling at various time intervals. This is demonstrated in Figure 2d, which shows a recirculating liquid-handling pump coupled to the LC injector that took samples intermittently from a stirred vial containing an analgesic tablet composed of acetaminophen, aspirin, and caffeine. As the tablet slowly dissolved over a period of 11 hours, the peak areas of the components grew as expected (Figures 2e and 2f). Over the full series of 50 chromatograms in this study, an overall retention time range of 3 s over the course of the experiment was observed for the caffeine peak, with an overall relative standard deviation for retention time under 1%. Such dissolution testing often requires manual or on-line sampling of larger volumes when traditional analytical-scale LC columns are used. In this capillary LC example, the relatively large number of injections (50) required 2 μL of sample over the course of the experiment (40 nL per run); this total volume is less than 10% of the volume of a single injection using sequential injection chromatography for a similar off-line experiment [25]. Thus, even for experiments conducted over longer time periods, the overall sample removed can be minimal. In the future, novel sampling approaches can be devised to provide improved coupling to process streams for on-line process monitoring using the portable capillary LC instrument.
2.3. Use of the Compact Capillary LC Platform for Forensic Separations
In addition to the advantage of a smaller footprint in laboratory settings for direct sampling from an adjacent vessel, another advantage of a portable LC system with an internal battery supply is that it can be operated remotely and utilized for field applications. This capability would be especially advantageous for the analysis of illicit drug compounds, both for on-site seized drug identification and point-of-care screening for substance abuse in clinical settings. Figure 3 depicts six relevant drug panels, including benzodiazepines, cannabinoids, stimulants (cocaine and metabolites), and opioids and opioid-related compounds. One challenge for this application area is the vast array of molar absorptivity values that exist for different drug classes, which can lead to lower sensitivity for compounds that do not absorb strongly at a given LED wavelength, for example as shown in Figures 3e and 3f in which lower signals are obtained even though the chemical concentrations are much higher than several of the other analytes shown. As previously reported, one solution to this issue is to combine multiple on-column LED-UV detectors that emit light at different wavelengths to provide absorbance values with higher signals for specific compounds [13]. Thus far, 255 nm and 275 nm have primarily been used, although other LED wavelengths can also be considered [26].
Figure 3.
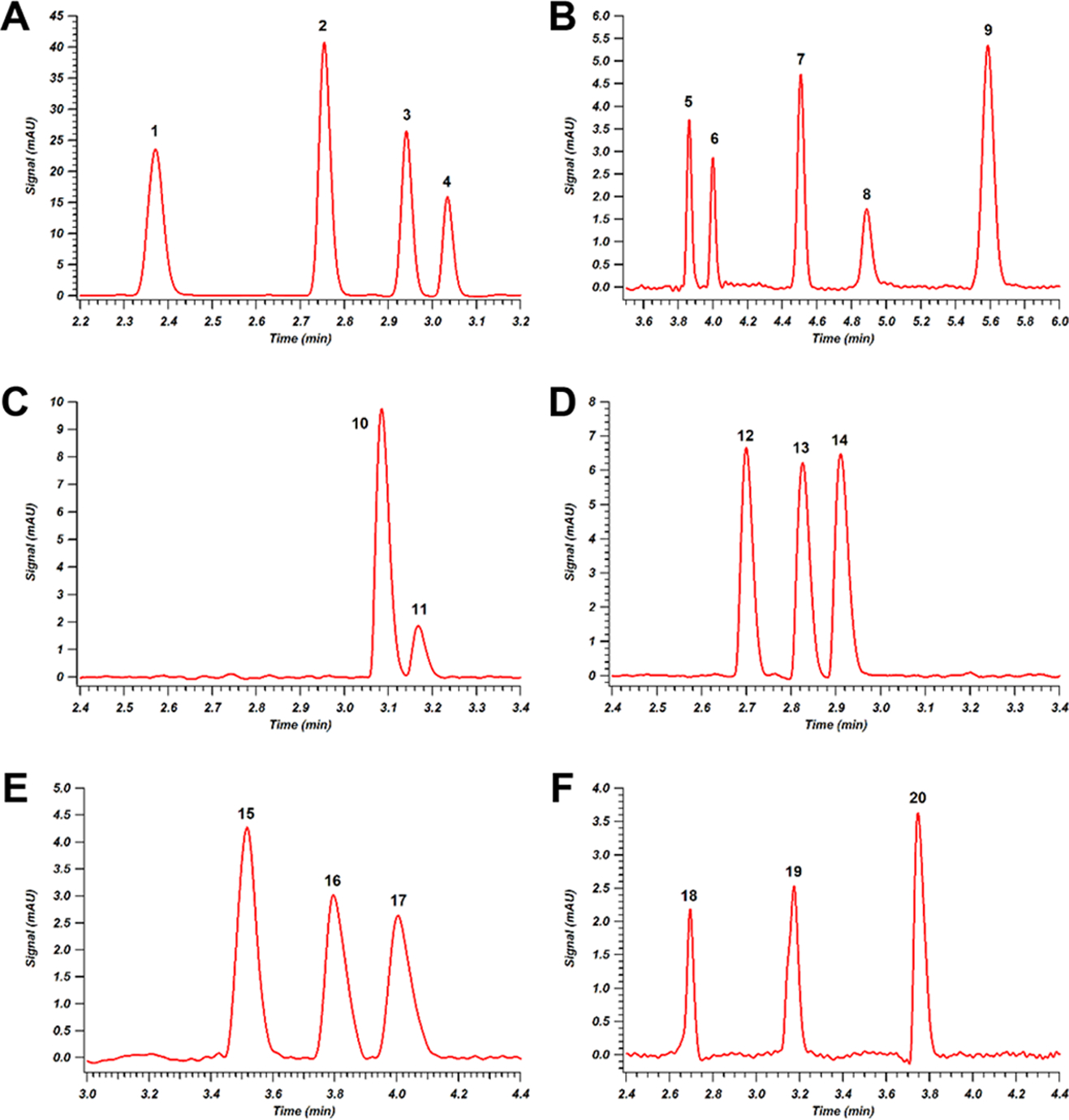
Chromatograms for various analytical panels of illicit drugs. Panel (A), benzodiazepines: (1) bromazepam, (2) nitrazepam, (3) diazepam, (4) lorazepam; Panel (B), cannabinoids: (5) cannabidiolic acid, (6) cannabidiol, (7) cannabinol, (8) Δ9-tetrahydrocannabinol, (9) Δ9-tetrahydrocannabinolic acid; Panel (C), methadone and metabolite: (10) 2-ethylidene-1,5-dimethyl-3,3-diphenylpyrrolidine (EDDP) perchlorate, (11) methadone; Panel (D), cocaine and metabolites: (12) benzoylecgonine, (13) cocaine, (14) cocaethylene; Panel (E), opioid drugs: (15) oxycodone, (16) hydrocodone, (17) codeine; Panel (F), heroin and metabolites: (18) morphine, (19) 6-acetylmorphine, (20) heroin. Further experimental details can be found in the Supporting Information.
When combining multiple LED-UV detectors in tandem at the column outlet in the format described above, an additional advantage is improved identification of target analytes through the use of dual-wavelength absorbance ratios for the detected compounds [27–30]. In this arrangement, both concentration and path length for each separated compound are identical at the adjacent detection points, but the molar absorptivity coefficients are different, depending on the wavelength of each LED. A ratio of two absorptivity coefficients can provide an analyte signature that provides more reliable identification than just retention time. Early results for a 4-compound test mixture using a prototype version of the detector with dual-wavelength absorbances at 255 nm and 275 nm were recently demonstrated [13]; continuing work is focused on using this approach with other wavelengths for the drug compounds described in this study.
3. Conclusions
The use of an integrated, portable capillary LC instrument for the analysis of pharmaceutical and illicit drug compounds was demonstrated for the first time. As shown in the data presented, applications that employ LC-UV methodology can be transferred to the new platform with flow rates that are 100–1,000 times lower than analytical-scale columns while maintaining similar separation performance. This “greener” approach to LC separations significantly reduces chemical usage and waste, a key driver toward more sustainable analytical techniques [31]. As this technology matures, it is likely that its use will expand for drug analysis and into other application areas that utilize LC-UV, and will be especially useful in field applications such as environmental testing. Work is ongoing to further develop methods utilizing this platform, as well as to further expand its capabilities into other chromatographic modes for even wider use.
Supplementary Material
Acknowledgements
Funding for this project was provided by the National Institutes of Health through award R41 DA045382. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Ray West and Greg Ward (Axcend Corporation) are acknowledged for technical assistance. Glenn Kresge and Alexis Zimmer (Rowan University) are acknowledged for helpful conversations related to this work.
Footnotes
Conflict of Interest Statement
XX, PAP, MLL, LTT, PBF and HDT are associated (either as employees or consultants) with the company (Axcend Corporation) that is developing the technology described in this manuscript for commercialization.
Bibliography
- [1].Mazzeo JR, D.Neue U, Kele M, Plumb RS, Advancing LC Performance with Smaller Particles and Higher Pressure. Anal. Chem 2005, 77, 460A–467A. [Google Scholar]
- [2].Yi L, Piehowski PD, Shi T, Smith RD, Qian WJ, Advances in microscale separations towards nanoproteomics applications. J. Chromatogr. A 2017, 1523, 40–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Chetwynd AJ, David A, A review of nanoscale LC-ESI for metabolomics and its potential to enhance the metabolome coverage. Talanta 2018, 182, 380–390. [DOI] [PubMed] [Google Scholar]
- [4].Wilson SR, Olsen C, Lundanes E, Nano liquid chromatography columns. Analyst 2019, 144, 7090–7104. [DOI] [PubMed] [Google Scholar]
- [5].Jorgenson JW, Capillary liquid chromatography at ultrahigh pressures. Annu. Rev. Anal. Chem 2010, 3, 129–150. [DOI] [PubMed] [Google Scholar]
- [6].Blue LE, Franklin EG, Godinho JM, Grinias JP, Grinias KM, Lunn DB, Moore SM, Recent advances in capillary ultrahigh pressure liquid chromatography. J. Chromatogr. A 2017, 1523, 17–39. [DOI] [PubMed] [Google Scholar]
- [7].Šesták J, Moravcová D, Kahle V, Instrument platforms for nano liquid chromatography. J. Chromatogr. A 2015, 1421, 2–17. [DOI] [PubMed] [Google Scholar]
- [8].Nazario CED, Silva MR, Franco MS, Lanças FM, Evolution in miniaturized column liquid chromatography instrumentation and applications: An overview. J. Chromatogr. A 2015, 1421, 18–37. [DOI] [PubMed] [Google Scholar]
- [9].Sharma S, Plistil A, Simpson RS, Liu K, Farnsworth PB, Stearns SD, Lee ML, Instrumentation for hand-portable liquid chromatography. J. Chromatogr. A 2014, 1327, 80–89. [DOI] [PubMed] [Google Scholar]
- [10].Sharma S, Plistil A, Barnett HE, Tolley HD, Farnsworth PB, Stearns SD, Lee ML, Hand-Portable Gradient Capillary Liquid Chromatography Pumping System. Anal. Chem 2015, 87, 10457–10461. [DOI] [PubMed] [Google Scholar]
- [11].Zhao X, Xie X, Sharma S, Tolley LT, Plistil A, Barnett HE, Brisbin M, Swensen AC, Price JC, Farnsworth PB, Tolley HD, Stearns SD, Lee ML, Compact Ultra-high Pressure Nano-flow Capillary Liquid Chromatograph. Anal. Chem 2017, 89, 807–812. [DOI] [PubMed] [Google Scholar]
- [12].Sharma S, Tolley HD, Farnsworth PB, Lee ML, LED-Based UV Absorption Detector with Low Detection Limits for Capillary Liquid Chromatography. Anal. Chem 2015, 87, 1381–1386. [DOI] [PubMed] [Google Scholar]
- [13].Xie X, Tolley LT, Truong TX, Tolley HD, Farnsworth PB, Lee ML, Dual-wavelength light-emitting diode-based ultraviolet absorption detector for nano-flow capillary liquid chromatography. J. Chromatogr. A 2017, 1523, 242–247. [DOI] [PubMed] [Google Scholar]
- [14].Sharma S, Tolley LT, Tolley HD, Plistil A, Stearns SD, Lee ML, Hand-portable liquid chromatographic instrumentation. J. Chromatogr. A 2015, 1421, 38–47. [DOI] [PubMed] [Google Scholar]
- [15].Li Y, Dvořák M, Nesterenko PN, Stanley R, Nuchtavorn N, Krčmová LK, Aufartová J, Macka M, Miniaturised medium pressure capillary liquid chromatography system with flexible open platform design using off-the-shelf microfluidic components. Anal. Chim. Acta 2015, 896, 166–176. [DOI] [PubMed] [Google Scholar]
- [16].Murray E, Li Y, Currivan SA, Moore B, Morrin A, Diamond D, Macka M, Paull B, Miniaturized capillary ion chromatograph with UV light-emitting diode based indirect absorbance detection for anion analysis in potable and environmental waters. J. Sep. Sci 2018, 41, 3224–3231. [DOI] [PubMed] [Google Scholar]
- [17].Chatzimichail S, Casey D, Salehi-Reyhani A, Zero electrical power pump for portable high-performance liquid chromatography. Analyst 2019, 144, 6207–6213. [DOI] [PubMed] [Google Scholar]
- [18].Lam SC, Coates LJ, Hemida M, Gupta V, Haddad PR, Macka M, Paull B, Miniature and fully portable gradient capillary liquid chromatograph. Anal. Chim. Acta 2019, DOI: 10.1016/j.aca.2019.12.014. [DOI] [PubMed] [Google Scholar]
- [19].Bui DA, Bomastyk B, Hauser PC, Absorbance detector based on a deep UV light emitting diode for narrow-column HPLC. J. Sep. Sci 2013, 36, 3152–3157. [DOI] [PubMed] [Google Scholar]
- [20].United States Pharmacopeial Convention, Naproxen Oral Solution. United States Pharmacop. Natl. Formul. (USP 40-NF 35) 2017, 5283–5284. [Google Scholar]
- [21].United States Pharmacopeial Convention, Ibuprofen. United States Pharmacop. Natl. Formul. (USP 40-NF 35) 2017, 4555–4556. [Google Scholar]
- [22].United States Pharmacopeial Convention, Acetaminophen and Aspirin Tablets. United States Pharmacop. Natl. Formul. (USP 40-NF 35) 2017, 8703–8704. [Google Scholar]
- [23].United States Pharmacopeial Convention, Chapter 621: Chromatography. United States Pharmacop. Natl. Formul. (USP 40-NF 35) 2017, 8071–8082. [Google Scholar]
- [24].Dolan JW, Method Adjustment the USP Way. LC-GC North Am. 2017, 35, 368–373. [Google Scholar]
- [25].Šatínský D, Neto I, Solich P, Sklenářová H, Conceição M, Montenegro BSM, Araújo AN, Sequential injection chromatographic determination of paracetamol, caffeine, and acetylsalicylic acid in pharmaceutical tablets. J. Sep. Sci 2004, 27, 529–536. [DOI] [PubMed] [Google Scholar]
- [26].Li Y, Nesterenko PN, Paull B, Stanley R, Macka M, Performance of a New 235 nm UV-LED-Based On-Capillary Photometric Detector. Anal. Chem 2016, 88, 12116–12121. [DOI] [PubMed] [Google Scholar]
- [27].Yost R, Stoveken J, MacLean W, Positive peak identification in liquid chromatography using absorbance ratioing with a variable-wavelength spectrophotometric detector. J. Chromatogr. A 1977, 134, 73–82. [DOI] [PubMed] [Google Scholar]
- [28].Drouen ACJH, Billiet HAH, De Galan L, Dual-wavelength absorbance ratio for solute recognition in liquid chromatography. Anal. Chem 1984, 56, 971–978. [Google Scholar]
- [29].White PC, Catterick T, Evaluation of absorbance ratioing for solute identification in high-performance liquid chromatography using a diode array detector. J. Chromatogr. A 1987, 402, 135–147. [Google Scholar]
- [30].White PC, Comparison of absorbance ratios and peak purity parameters for the discrimination of solutes using high-performance liquid chromatography with multi-wavelength detection. Analyst 1988, 113, 1625–1629. [Google Scholar]
- [31].Keith LH, Gron LU, Young JL, Green analytical methodologies. Chem. Rev 2007, 107, 2695–2708. [DOI] [PubMed] [Google Scholar]

/Website/Focus%20LC%20(w%20label).png?width=810&height=688&name=Focus%20LC%20(w%20label).png)
/Website/AutoFocus%20(w%20label).png?width=810&height=743&name=AutoFocus%20(w%20label).png)
/Website/InFocus%20(w%20label).png?width=810&height=746&name=InFocus%20(w%20label).png)
/Website/FocusArray%20(w%20label).png?width=810&height=556&name=FocusArray%20(w%20label).png)
/Website/imgnav-focusanalyze.png?width=426&height=289&name=imgnav-focusanalyze.png)
/Website/lc-robust.jpg?width=433&height=400&name=lc-robust.jpg) Targeted peptide quantification with small foot-print capillary LC-MS/MS
Targeted peptide quantification with small foot-print capillary LC-MS/MS
 See us at IFPAC March 2-4! Booth 503
See us at IFPAC March 2-4! Booth 503
/Website/FA%202.png?width=800&height=729&name=FA%202.png) Axcend® announces the launch of its small footprint, full-stack chromatography system.
Axcend® announces the launch of its small footprint, full-stack chromatography system.